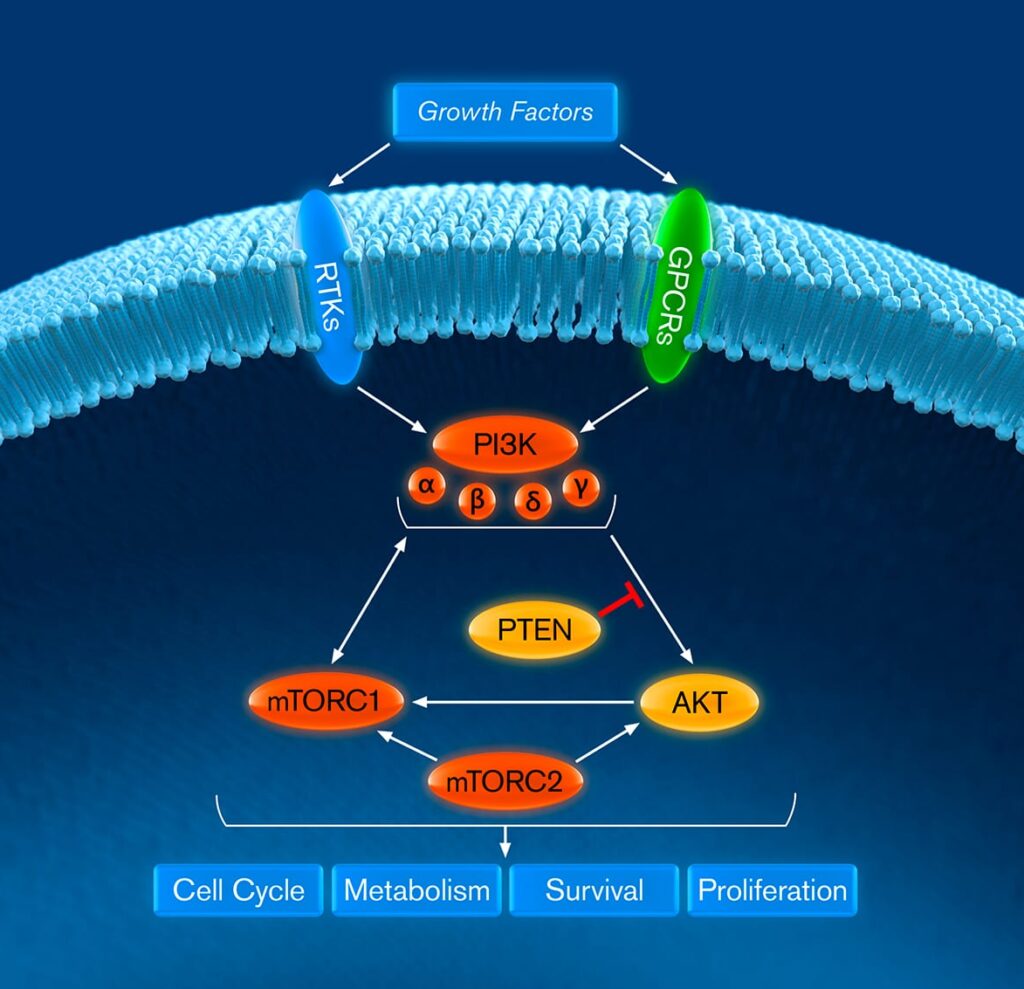
Our lead drug candidate, gedatolisib, is a potential first-in-class pan-PI3K and mTORC1/2 inhibitor
Our CELsignia platform allows us to obtain proprietary insights about the relative effectiveness of targeted therapies. Our ex vivo studies found that gedatolisib inhibited higher levels of PI3K/AKT/mTOR involved signaling activity than single target PI3K, AKT, or mTOR targeted therapeutics, regardless of PIK3CA mutational status, and demonstrated superior drug synergy when combined with other targeted therapies.
Gedatolisib’s mechanism of action and pharmacokinetic properties are highly differentiated from other currently approved and investigational therapies that target PI3K, AKT or mTOR alone. We believe this may enable gedatolisib to treat a broader patient population than these other inhibitors.
Gedatolisib’s initial clinical development program is focused on the treatment of patients with estrogen receptor positive (ER+), human epidermal growth factor receptor 2 -negative (HER2-), advanced or metastatic breast cancer and patients with metastatic castration resistant prostate cancer. Unlike PI3K or AKT therapies that are only approved to treat patients with PIK3CA or PTEN mutations, gedatolisib is under development for patients with and without PIK3CA mutations.
Gedatolisib
Learn more