VIKTORIA-1
A Phase 3 trial evaluating gedatolisib as a 2nd-line treatment for HR+/HER2- advanced breast cancer
VIKTORIA-1 is a Phase 3 study evaluating gedatolisib plus fulvestrant with and without palbociclib in patients previously treated with a CDK4/6 therapy and an aromatase inhibitor.
Approximately 701 eligible patients whose PIK3CA mutational status has been determined will be enrolled.
Eligible patients who do not have confirmed PI3KCA mutations (WT) will be randomly assigned (1:1:1) to receive a regimen of either gedatolisib, palbociclib, and fulvestrant (Arm A); gedatolisib and fulvestrant (Arm B); or fulvestrant (Arm C).
Eligible patients who have confirmed PI3KCA mutations (MT) will be randomly assigned (3:3:1) to receive a regimen of either gedatolisib, palbociclib, and fulvestrant (Arm D); alpelisib and fulvestrant (Arm E); or gedatolisib and fulvestrant (Arm F).

Rationale
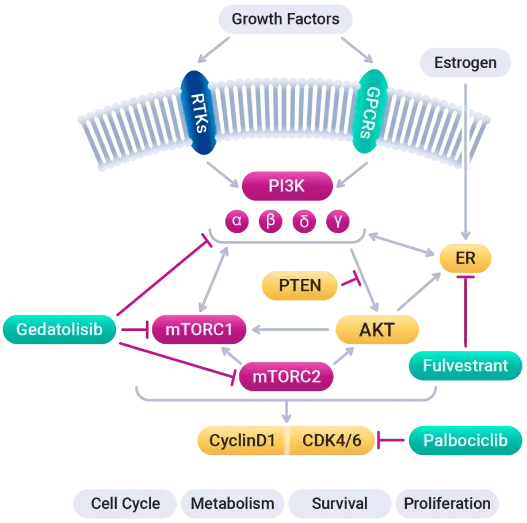
Simultaneous inhibition of the PAM (PI3K/AKT/mTOR) pathway with gedatolisib, the CDK4/6 pathway with palbociclib, and the estrogen receptor pathway with fulvestrant, is intended to disrupt complex cooperation between these pathways to inhibit tumor growth.
Available evidence indicates that resistance to CDK4/6 inhibition is a transient adaptive mechanism, most likely involving the PAM pathway. Continuing CDK4/6 inhibition in combination with PAM inhibition in patients who progressed on their prior CDK4/6 inhibitor is expected to both block the reactivated CDK4/6 pathway and prevent adaptive activation of the PAM pathway.
Patients whose disease progressed on a CDK4/6 inhibitor may thus potentially benefit from continued treatment with a CDK4/6 and ER inhibitor when it is combined with a PAM inhibitor as their next line of therapy.
Key eligibility criteria
- Locally advanced or metastatic ER+, HER2- breast cancer
- Confirmed PIK3CA mutation status
- Prior treatment with a CDK4/6 inhibitor combined with an aromatase inhibitor
- No prior treatment with AKT, PI3K, mTOR inhibitors
- No prior chemotherapy for advanced disease
- No more than 2 prior lines of endocrine therapy treatment for metastatic disease
Key endpoints
- Arm A vs. Arm C
- Arm B vs. Arm C
- Arm D vs. Arm E
- Overall survival
- Overall response rate
- Duration of response
Study locations
VIKTORIA-1 is being conducted at sites in North America, Europe, South America, Australia, and Asia.