Prostate Cancer
A Phase 1/2 clinical trial evaluating gedatolisib for patients with metastatic castration resistant prostate cancer

The CELC-G-201 Phase 1/2 clinical trial is evaluating gedatolisib plus darolutamide, an androgen receptor (AR) inhibitor, in patients previously treated with an AR inhibitor for metastatic castration resistant prostate cancer (mCRPC).
Approximately 48 to 54 eligible patients will be enrolled.
In the Phase 1 portion of the study, 18 participants will be randomly assigned to each of 2 dose arms (n = 36 total) and evaluated in two parts. Arm 1 will evaluate 120 mg gedatolisib, and Arm 2 will evaluate 180 mg gedatolisib.
In the Phase 2 study, an additional 12 participants will then be enrolled at the recommended Phase 2 dose (RP2D) level. The total number of participants will be approximately 48 to 54, with 30 participants enrolled at the RP2D level.
Rationale
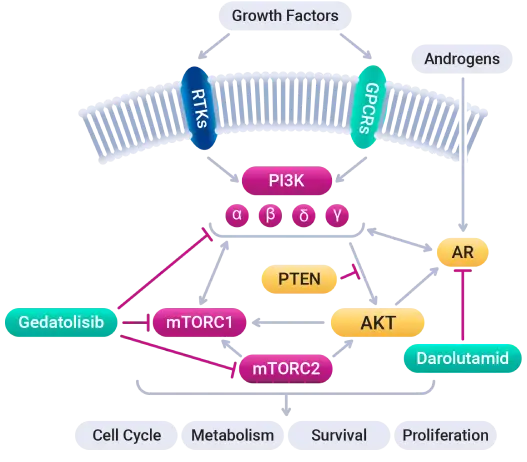
Simultaneous inhibition of the PAM (PI3K/AKT/mTOR) pathway with gedatolisib and the AR pathway with darolutamide is intended to disrupt complex cooperation between these pathways to inhibit tumor growth.
The cancer driving PAM pathway is activated in the majority of mCRPC tumors. Since the PAM and AR pathways cross-regulate each other through reciprocal negative feedback, resistance to AR inhibition can be induced through the activation of PI3K/AKT/mTOR signaling.
Patients whose disease progressed on an AR inhibitor may thus potentially benefit from continued treatment with an AR inhibitor when it is combined with a PAM inhibitor like gedatolisib.
Key eligibility criteria
- Metastatic castration resistant prostate cancer
- Prior treatment with an AR inhibitor
- No prior treatment with AKT, PI3K, mTOR inhibitors
- No prior chemotherapy for mCPRC
Key endpoints
- Safety
- Recommended Phase 2 dose
- Radiographic progression-free survival at six months
- Radiographic progression-free survival at 12 months
- Overall response rate
Study locations
CELC-G-201 is being conducted at sites in the United States and Europe.